lithium valence electrons|Lithium (Li) : Tuguegarao Mar 23, 2023 We would like to show you a description here but the site won’t allow us.
PH0 · What Are Valence Electrons? Definition and Periodic
PH1 · Valences of the Chemical Elements
PH2 · Valence Electrons Chart for All Elements
PH3 · Lithium Valence Electrons
PH4 · Lithium (Li)
PH5 · Khan Academy
PH6 · How to Find the Valence Electrons for Lithium (Li)
PH7 · Chemistry of Lithium (Z=3)
PH8 · 3.10: Valence Electrons
PH9 · 2.9: Valence Electrons
bet365 Radio Commentary. #AD – Access the latest bet365 radio commentary. About bet365 Radio bet365 radio features [.] Is bet365 down? Find out whether the bet365 website is up or down. Bet365 Horse Racing Best Odds. We believe that Bet365 is arguably the world’s leading bookmaker when it comes to [.]
lithium valence electrons*******Mar 23, 2023 Learn how to identify valence electrons, the outer-shell electrons that determine the reactivity of an atom. See examples of valence electrons for representative and .lithium valence electrons Lithium (Li) You may assume the valences of the chemical elements—the number of . There are two ways to find the number of valence electrons in Lithium (H). The first is to use the Periodic Table to figure out how many electrons Lithium has in its . Learn about the properties, trends, reactivity, and applications of lithium, the lightest and least dense metal. Lithium has one valence electron and forms compounds .Lithium (Li) Learn how to identify and count valence electrons, the electrons in the outermost shell of an atom, with examples and explanations. See how valence electrons affect chemical . Learn how to identify valence electrons using the periodic table and electron configuration. Lithium has one valence electron in the 2s sublevel, while sodium has one .
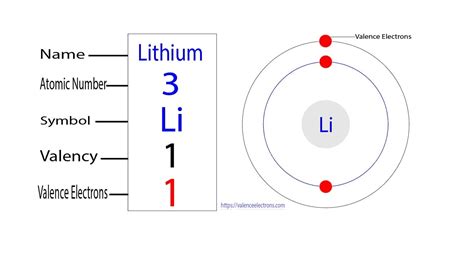
Lithium has three protons and four neutrons in its nucleus, and three electrons in two shells. It is located in group one, period two and block s of the periodic table. Socket . It always reacts with the water. The most important use of lithium is in the rechargeable batteries for mobile phones, laptops, digital cameras, etc. As well as they are used in non-rechargeable batteries . Learn what valence electrons are and how to find them for different elements. Lithium has one valence electron and an oxidation state of +1.Element Lithium (Li), Group 1, Atomic Number 3, s-block, Mass 6.94. Sources, facts, uses, scarcity (SRI), podcasts, alchemical symbols, videos and images. . Members of a group typically have similar properties and electron configurations in their outer shell. Period A horizontal row in the periodic table. The atomic number of each element . Therefore, the lithium complete electron configuration will be 1s 2 2s 1. Note: The unabbreviated electron configuration of lithium is [He] 2s 1. Electron configuration for Lithium-ion (Li +) After the . Lithium has a single electron in the second principal energy level and so we say that lithium has one valence electron. Beryllium has two valence electrons. How many valence electrons does boron have? You must recognize that the second principal energy level consists of both the \(2s\) and the \(2p\) sublevels and so the answer is three.
For example, a lithium atom has 1 valence electron and has an oxidation state of +1. In contrast, a neon atom has 8 valence electrons and an oxidation state of 0. A hydrogen atom has 1 valence electron. It has an oxidation state of +1 when it combines with most elements, but an oxidation state of -1 when it forms a compound with an alkali .
Here, we have mentioned the Lewis structure of the lithium. The lithium valence electrons dot diagram is as shown here: lithium – 1 s 2 2 s 1 – 1 valence electron. What is this electrons dot diagram is made up of? Electrons is the atom’s outmost energy level are the elections that are important in chemical bond and . When filling the p orbitals, each takes a single electron; once each p orbital has an electron, a second may be added. Lithium (Li) contains three electrons that occupy the first and second shells. Two electrons fill the 1s orbital, and the third electron then fills the 2s orbital. Its electron configuration is 1s 2 2s 1. Khanmigo is now free for all US educators! Plan lessons, develop exit tickets, and so much more with our AI teaching assistant.Valence electrons are also the determining factor in some physical properties of the elements. Elements in any one group (or column) have the same number of valence electrons; the alkali metals lithium and sodium each have only one valence electron, the alkaline earth metals beryllium and magnesium each have two, and the halogens . The next atom, lithium, has an electron configuration of 1s 2 2s 1, so it has only one electron in its valence shell. Its electron dot diagram resembles that of hydrogen, except the symbol for lithium is used: \[\mathbf{Li}\mathbf{\cdot}\nonumber \] Beryllium has two valence electrons in its 2s shell, so its electron dot diagram is like that of . There are two ways to find the number of valence electrons in Lithium (H). The first is to use the Periodic Table to figure out how many electrons Lithium ha.
Lithium has a single electron in the second principal energy level, and so we say that lithium has one valence electron. Beryllium has two valence electrons. How many valence electrons does boron have? Recognize that the second principal energy level consists of both the \(2s\) and the \(2p\) sublevels, and so the answer is three.
The electrons that determine valence – how an atom reacts chemically – are those with the highest energy.. For a main-group element, the valence electrons are defined as those electrons residing in the electronic shell of highest principal quantum number n. [1] Thus, the number of valence electrons that it may have depends on the electron .

In the case of lithium, its valence electron is the single electron in the 2s subshell of the second shell. The valence electron in lithium is loosely bound to the nucleus, which makes it highly reactive and easily lost. This reactivity is why lithium is an excellent material for use in batteries, as it can donate its valence electron to other .
lithium valence electronsLithium's lower reactivity is due to the proximity of its valence electron to its nucleus (the remaining two electrons are in the 1s orbital, much lower in energy, and do not participate in chemical bonds). [10] . As an exception to the duet rule, a two-coordinate lithate complex with four electrons around lithium, [Li . Valence electrons are the electrons that reside in the outermost energy level of an atom and are, therefore, the most accessible for the formation of chemical bonds. The number of valence electrons in one atom of each element is easily determined based on its position in the periodic table. For the main group elements (groups designated .
Les électrons de valence pour un lithium-ion ont deux électrons puisque la coque qui contient la dernière coque lithium-ion a deux électrons. Quelle est la valence du lithium ? La valence (ou valence) est la capacité de lier un atome et de créer des composés. Il existe quelques règles qui peuvent être utilisées pour déterminer la .Assigning Electron Configuration . We write electronic configurations by following the aufbau principle (from German, meaning “building up”). First we determine the number of electrons in the atom; then we add electrons one at a time to the lowest-energy orbital available without violating the Pauli Exclusion Principle .That is, recognizing that each .Valence electrons are the electrons present in the outermost shell of an atom. You can easily determine the number of valence electrons an atom can have by looking at its Group in the periodic table. For example, atoms in Groups 1 and 2 have 1 and 2 valence electrons, respectively. Atoms in Groups 13 and 18 have 3 and 8 valence electrons .
Other experts state that 3, 9, and 2 are the luckiest numbers for Aquarius. As you can see 9 seems to be a recurring number so you may want to use this number to your advantage. There are other lucky factors for Aquarius (Kumbh Rashi) such as colors, days of the week, and gemstones.. With that being said, sky blue is the luckiest color for this .
lithium valence electrons|Lithium (Li)